Are you waking up every morning with shoulder pain? Your bed might be to blame. Plenty of sleepers, but especially those who prefer to sleep on their side, suffer from this experience. An uncomfortable mattress that lets pressure build up instead of relieving it can only exacerbate the problem.
To help prevent this issue, we’ve put together a list of our recommendations for the best mattresses for shoulder pain. All of these mattresses are constructed with cushioning and support in mind, but we’ve narrowed down the choices to address your specific needs. Read on to find out which bed is the best fit for you.
Best Mattresses for Shoulder Pain
- Puffy Royal Hybrid – Editor’s Pick
- Nectar – Best Memory Foam Mattress for Shoulder Pain
- Helix Midnight Luxe – Best Mattress for Side Sleepers with Shoulder Pain
- WinkBed – Best Mattress for Neck and Shoulder Pain
- Titan Plus Luxe – Best Mattress for Heavy People with Shoulder Pain
- Saatva Classic – Best Mattress for Back and Shoulder Pain
- Bear Elite Hybrid – Best Hybrid Mattress for Shoulder Pain
- Nolah Signature 12″ – Best Soft Mattress for Shoulder Pain
- Brooklyn Bedding Sedona Elite – Best Firm Mattress for Shoulder Pain
- Birch Natural – Best Organic Mattress for Shoulder Pain
Compare the Best Mattresses for Shoulder Pain

|

|

|

|

|

|

|

|

|

|
|
| Puffy Royal Hybrid Mattress | Nectar Mattress | Helix Midnight Luxe Mattress | WinkBed Mattress | Brooklyn Bedding Titan Plus Luxe | Saatva Mattress | Bear Elite Hybrid Mattress | Nolah Signature 12″ Mattress | Brooklyn Bedding Sedona Elite Mattress | Birch Natural Mattress | |
| Rating | ||||||||||
| Firmness | Medium: 5.5/10 | Medium-firm: 6.5/10 | Medium-firm: 6/10 | Multiple firmness options | Medium-firm: 6.5/10 | Multiple firmness options | Medium-firm: 6/10 | Soft: 4/10 | Medium: 5.5/10 | Medium-firm: 6.5/10 |
| Material | Hybrid | Foam | Hybrid | Hybrid | Hybrid | Innerspring | Hybrid | Foam | Hybrid | Hybrid |
| Cooling | — | — | — | — | — | |||||
| Warranty | Lifetime warranty | Lifetime warranty | 15-year warranty | Lifetime warranty | 10-year warranty | Lifetime warranty | Lifetime Warranty | Lifetime warranty | 10-year warranty | 25-year warranty |
| Shipping | Free shipping | Free shipping | Free shipping | Free shipping | Free shipping minus HI and AK | Free white glove delivery | Free shipping | Free shipping | Free shipping | Free shipping |
| Trial Period | 101 nights | 365 nights | 100 nights | 120 nights | 120 nights | 365 nights | 120 nights | 120 nights | 120 nights | 100 nights |
| Best For | Side Sleepers, Back Sleepers, Couples, Back Pain | Back Sleepers, Side Sleepers, Hip Pain, Joint Pain | Back Sleepers, Stomach Sleepers, Side Sleepers, Hip Pain, Seniors | Back Sleepers, Stomach Sleepers, Back Pain, Hot Sleepers, Side Sleepers, Hip Pain, Seniors | Side Sleepers, Back Sleepers | Back Sleepers, Stomach Sleepers, Back Pain, Hot Sleepers, Seniors | Back Sleepers, Stomach Sleepers, Hot Sleepers, Hip Pain, Seniors | Side Sleepers, Back Pain | Side Sleepers, Hot Sleepers, Couples | Back Sleepers, Stomach Sleepers, Hot Sleepers, Seniors |
Sleep Advisor’s Testing Methodology
When we test mattresses, there are certain performance qualities we asses, including support, pressure relief, and ease of mobility — among others. Our hands-on testing process allows us to see what types of sleepers could benefit from certain beds based on their performance.
In the case of good mattresses for shoulder pain, we are looking at specific qualities that will be more important and valuable for someone navigating pain in this area and trying to get a good night’s rest.
For more on our testing methodology, visit our in-depth product review process.

Advice from a Medical Expert
We reached out to Dr. Raj Dasgupta, who has over 20 years of experience in the medical field, to see his thoughts on what key qualities people with shoulder pain should look for in a mattress.
“To ease shoulder pain, look for a mattress that gives good support to keep your spine aligned while also cushioning your shoulders. Aim for something not too soft or too firm, like a medium mattress with memory foam or latex.”
Puffy Royal Hybrid – Editor’s Pick
Puffy Royal Hybrid Mattress

A soft, luxurious mattress that offers deep sinkage and support to side and back sleepers.
Material
Hybrid
Trial Period
101 nights
Shipping Method
Free shipping
Firmness
Medium: 5.5/10
Warranty
Lifetime warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Puffy Royal Hybrid Mattress

A soft, luxurious mattress that offers deep sinkage and support to side and back sleepers.
Material
Hybrid
Warranty
Lifetime warranty
Firmness
Medium: 5.5/10
Shipping Method
Free shipping
Trial Period
101 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.

Puffy Royal Hybrid Mattress
A soft, luxurious mattress that offers deep sinkage and support to side and back sleepers.
Material
Hybrid
Firmness
Medium: 5.5/10
Trial Period
101 nights
Warranty
Lifetime warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Why the Puffy Royal Hybrid Earned Best Mattress for Shoulder Pain Overall
If you’re waking up with shoulder pain, the cause could be a too-firm mattress. Enter the Puffy Royal Hybrid—I rated this bed a 4 out of 10 on our firmness scale, meaning it is plush with a capital P for ultimate cushioning capability.
You should sink right into this bed and experience very little pressure buildup, if any. The foam has a pretty decent spring-back, too, so it should feel relatively easy to switch positions. For these reasons, we’ve given the Puffy Royal Hybrid our top pick for a bed that might nip shoulder pain in the bud.

What’s the Puffy Royal Hybrid Made Of?
The Puffy Royal Hybrid is 14 inches thick with seven total layers.
Inside the Puffy Royal Hybrid
- Layer 1 – Stain-Resistant Cloud Cover. A hypoallergenic, removable cover.
- Layer 2 – Gel-Infused Cooling Foam (1.5 inches). Gel keeps this contouring foam cool while it conforms to your body.
- Layer 3 – Reflexive Memory Foam (1.5 inches). This foam bounces back into shape more quickly than traditional memory foams for responsive pressure relief.
- Layer 4 – Climate Comfort™ Foam (2 inches). A humidity-resistant foam layer ups the cooling capability of this mattress and keeps air flowing through the bed.
- Layer 5 – Cloud Air Technology Foam (2 inches). This foam layer is zoned into five distinct areas to cushion and support different parts of the body properly.
- Layer 6 – Firm Core Support Foam and Contour-Adapt Coils (7 inches). Dense foam and coils work together in this layer to keep you lifted while also dampening motion transfer.
- Layer 7 – Grip Base Cover. The bottom cover keeps the mattress in place.
My Take: The Puffy Royal Hybrid is sumptuously soft, so it should cradle problem areas with that enviable “hug” offered by memory foam. I think side sleepers should feel little to no pressure buildup in the shoulder area sleeping on this bed.
What I Liked
- Stellar motion isolation – Usually hybrids are too springy to allow for major motion isolation, but the thick and comfy foam layers of the Puffy Royal Hybrid absorb movement very well.
- Serious pressure relief – These same foams also provide deep pressure relief. You should sink into its cloud-like profile and feel all your sensitive joints cradled by the specialized layers.
Potential Drawbacks
- Could be cooler – Despite many of the foam layers offering cooling technology in their designs, I wasn’t exactly impressed by the Puffy Royal Hybrid’s ability to regulate temperature. If you’re an especially hot sleeper, consider a mattress with less heat-trapping foams.
- Best suited for side sleepers – The Puffy Royal Hybrid’s softness is best suited to side sleepers. Some back sleepers may be able to find the right level of support, but for stomach sleepers, it’s a no-go. Heavyweight sleepers may also struggle to stay lifted on the bed’s plush surface.
Want to know more? Check out our Puffy Royal Hybrid review or our picks for the best mattresses in 2024.
Nectar – Best Memory Foam Mattress for Shoulder Pain
Nectar Mattress

Nectar offers the deeper hug of a traditional memory foam bed but with updated cooling and a Forever warranty.
Material
Foam
Trial Period
365 nights
Shipping Method
Free shipping
Firmness
Medium-firm: 6.5/10
Warranty
Lifetime warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Joint Pain
This bed is perfect for anyone suffering from joint pain.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Nectar Mattress

Nectar offers the deeper hug of a traditional memory foam bed but with updated cooling and a Forever warranty.
Material
Foam
Warranty
Lifetime warranty
Firmness
Medium-firm: 6.5/10
Shipping Method
Free shipping
Trial Period
365 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Joint Pain
This bed is perfect for anyone suffering from joint pain.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.

Nectar Mattress
Nectar offers the deeper hug of a traditional memory foam bed but with updated cooling and a Forever warranty.
Material
Foam
Firmness
Medium-firm: 6.5/10
Trial Period
365 nights
Warranty
Lifetime warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Joint Pain
This bed is perfect for anyone suffering from joint pain.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Why the Nectar Earned Best Memory Foam Mattress for Shoulder Pain
An all-foam mattress is one of your best bets if you’re looking to mitigate shoulder pain. The Nectar, one such option, stands out because of its price, its policies, and, most importantly, its balanced profile.
Softer foams on top ensure you get all the pressure relief you need while firmer foams below keep you supported. This combination should benefit sleepers with aches and pains, keeping them in that sweet spot for comfortable sleep throughout the night.

What’s the Nectar Made Of?
The Nectar is 12 inches tall. It has three foam layers plus two covers for a total of five layers. It uses only CertiPUR-US® certified foams in its construction.
Inside the Nectar
- Layer 1 – Top Cover. Polyethylene fibers give the Nectar a cooler sleep surface.
- Layer 2 – Gel Memory Foam (2 inches). This pressure-relieving memory foam layer is infused with gel to help it feel cooler than typical foams.
- Layer 3 – Dynamic Response Foam (3 inches). This foam will bounce back in response to your movements while keeping air flowing through the mattress.
- Layer 4 – ActiveSupport Foam Base (7 inches). A thick foam base is the foundation for the Nectar mattress.
- Layer 5 – Lower Cover. A bottom cover ensures there’s no unwanted shifting.
My Take: The Nectar mattress checks off plenty of boxes. If you’re on a budget and looking for quality, it’s a near-perfect fit. Back and side sleepers should rest easy knowing their sensitive joints are cradled by its soft surface.
What I Liked
- Nice price and policies – A queen Nectar mattress usually retails for under $700, thanks to frequent sales. Not only is it a great deal, but you get a year to try it out, along with a lifetime warranty.
- Soft but little sink – The firmer foam foundation underneath the comfort layers allows this bed to provide a comfortable sink—but not so much of one that you feel uneven.
Potential Drawbacks
- Too soft for some – Unfortunately, there are a few sleepers I wouldn’t recommend the Nectar for: mainly heavier folks and stomach sleepers. This bed is still too soft to give these groups the proper support. As a stomach sleeper myself, I noticed some uncomfortable sink around my midsection in the testing studio.
- Not the coolest – Even the best cooling innovations can’t trump the heat-retaining qualities of an all-foam mattress. That’s the case for the Nectar—it’s not the best choice for hot sleepers.
Interested in learning more? Read our Nectar mattress review or our article on the best memory foam mattresses.
Helix Midnight Luxe – Best Mattress for Side Sleepers with Shoulder Pain
Helix Midnight Luxe Mattress

With the Ultra-Cool phase-changing cover and gel-infused foam, the Helix Midnight Luxe is an excellent choice for hot sleepers who also need pressure relief and zoned support.
Material
Hybrid
Trial Period
100 nights
Shipping Method
Free shipping
Firmness
Medium-firm: 6/10
Warranty
15-year warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for average weight and heavyweight back sleepers.Side Sleeping
Ideal for average weight and heavyweight side sleepers.Stomach Sleeping
Ideal for lightweight stomach sleepers.Financing Options
Financing options are available for this mattress.
Helix Midnight Luxe Mattress

With the Ultra-Cool phase-changing cover and gel-infused foam, the Helix Midnight Luxe is an excellent choice for hot sleepers who also need pressure relief and zoned support.
Material
Hybrid
Warranty
15-year warranty
Firmness
Medium-firm: 6/10
Shipping Method
Free shipping
Trial Period
100 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for average weight and heavyweight back sleepers.Side Sleeping
Ideal for average weight and heavyweight side sleepers.Stomach Sleeping
Ideal for lightweight stomach sleepers.Financing Options
Financing options are available for this mattress.

Helix Midnight Luxe Mattress
With the Ultra-Cool phase-changing cover and gel-infused foam, the Helix Midnight Luxe is an excellent choice for hot sleepers who also need pressure relief and zoned support.
Material
Hybrid
Firmness
Medium-firm: 6/10
Trial Period
100 nights
Warranty
15-year warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for average weight and heavyweight back sleepers.Side Sleeping
Ideal for average weight and heavyweight side sleepers.Stomach Sleeping
Ideal for lightweight stomach sleepers.Financing Options
Financing options are available for this mattress.
Why the Helix Midnight Luxe Earned Best Mattress for Side Sleepers with Shoulder Pain
The Helix Midnight Luxe is Helix’s luxury bed designed for side sleepers. Its plush pillow top should feel like a dream for anyone dealing with shoulder pain, as its quilted foams provide top-notch pressure relief.
Its pain-relieving ability is bolstered by the layers beneath: specialized foams and a zoned coil unit offer crucial support, keeping joint areas.

What’s the Helix Midnight Luxe Made Of?
The Helix Midnight Luxe is composed of six layers and is 13.5 inches tall. It uses CertiPUR-US® certified foams in its construction.
Inside the Helix Midnight Luxe
- Layer 1 – Cooling Pillow Top. Choose the standard Tencel™ cover for natural breathability or upgrade to a cooling GlacioTex™ cover. The pillow top itself is made up of an inch of quilted foam for cushioning at the mattress surface.
- Layer 2 – Helix Responsive Foam. More responsive than traditional memory foam, this layer quickly adapts to your weight and movements.
- Layer 3 – Copper Gel Memory Foam. Copper is naturally cooling and antibacterial, giving this pressure-relieving foam layer a cooler, cleaner profile.
- Layer 4 – Memory Plus Foam. A dense foam layer supports the comfort layers while transitioning into the coil layer.
- Layer 5 – Spring Unit. Individually wrapped coils respond to your movements while special zoning in the center of the mattress targets the lumbar area with increased support. The coils around the perimeter are also reinforced.
- Layer 6 – DuraDense Foam. A dense foam foundation.
Our Take: “We use a heat map that shows pressure distribution and found that [the Helix Midnight Luxe] evenly distributes your weight, which helps keep pressure and pain off of the hips and shoulders of side sleepers. It also kept us aligned, so I’ll agree [with the brand] and say that this is a good choice for side sleepers.” – Loren Bullock, Lead Product Tester
What We Liked
- Versatile – The Helix Midnight Luxe, though designed for side sleepers, has a medium-firm feel that should work for a variety of sleepers and preferred positions. Anyone looking for a cushy-but-supportive luxury mattress may want to consider the Midnight Luxe.
- Robust edges – Reinforcement in the mattress’s perimeter gives this bed an edge—that is, a strong one. You should be able to make full use of the mattress’s surface area, sitting or sleeping near the edges.
Potential Drawbacks
- Luxurious price – The price certainly reflects this mattress’s high quality. A queen will cost you over $2,000 before sales—definitely worth it, but definitely not a budget option.
- Not for stomach sleepers – Though the Helix Midnight Luxe can work for a variety of folks, as a stomach sleeper, I wasn’t totally sold. Unless you’re a petite sleeper, you’re probably going to sink too far into this bed.
Still curious? Check out our dedicated Helix Midnight Luxe review, or peruse the best mattresses for side sleepers.
WinkBed – Best Mattress for Neck and Shoulder Pain
WinkBed Mattress

WinkBed is a luxury hybrid bed with a variety of comfort features that should make it a versatile pick for most sleeping positions.
Material
Hybrid
Trial Period
120 nights
Shipping Method
Free shipping
Firmness
Multiple firmness options
Warranty
Lifetime warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
WinkBed Mattress

WinkBed is a luxury hybrid bed with a variety of comfort features that should make it a versatile pick for most sleeping positions.
Material
Hybrid
Warranty
Lifetime warranty
Firmness
Multiple firmness options
Shipping Method
Free shipping
Trial Period
120 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.

WinkBed Mattress
WinkBed is a luxury hybrid bed with a variety of comfort features that should make it a versatile pick for most sleeping positions.
Material
Hybrid
Firmness
Multiple firmness options
Trial Period
120 nights
Warranty
Lifetime warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight and average weight back sleepers.Side Sleeping
Ideal for lightweight and average weight side sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Why the WinkBed Earned Best Mattress for Neck and Shoulder Pain
Shoulder and neck pain can be an unfortunate pairing, but a bed that promotes good spinal posture can help. Thankfully, the WinkBed is a sturdy hybrid bed with a lifted profile that should keep everything properly elevated and aligned.
It also comes in four different firmness models, including a specialized Plus model for plus-sized sleepers. You can tailor the bed to your specific needs, which can aid in pain management.

What’s the WinkBed Made Of?
The WinkBed comes in four firmnesses: Soft, Luxury Firm, Firm, and Plus. Each model is about 13 inches tall.
Inside the WinkBed
- Tencel™ Cover – Tencel™ fibers make for a breathable and moisture-free sleep surface.
- Euro Pillow Top – Quilted gel-infused foams provide cooling pressure relief and cushioning at the very top of the mattress.
- Intermediary Foam Layers – In between the pillow top and the innerspring unit are a series of foam layers to bolster support.
- Coils – The coils in this layer are zoned to ensure your body is optimally positioned with the right balance of lift and give.
Our Take: “The mattress is very bouncy and buoyant, and the pillow top layer adds a luxurious level of comfort while the coils keep your body mostly elevated on the mattress.” – Loren, Lead Product Tester
What We Liked
- Plenty of options – With three firmnesses ranging from soft to firm, and a specialized “Plus” model, it should be easy to find your match based on your body type and sleeping position. If you’re looking for a true middle-of-the-road feel that should suit a variety of sleepers, we recommend the Luxury Firm.
- Sturdy – WinkBed has independently tested its springs to ensure they won’t sag after repeated use. You should get plenty of years of use from this bed.
Potential Drawbacks
- Not super cradling – The WinkBed does have foam comfort layers, but they won’t rival the deep sink of traditional memory foam. You’ll feel more like you’re sleeping on top of this mattress rather than getting hugged by it.
- Some motion carries – During testing, Loren and I noticed that movement carries across the mattress surface, so the WinkBed may not be the best choice for light sleepers who share the bed.
Need to know more? See our full WinkBed review or the best mattresses for neck pain.
Titan Plus Luxe – Best Mattress for Heavy People with Shoulder Pain
Brooklyn Bedding Titan Plus Luxe

This specially-designed hybrid is made for heavier sleepers to help them get better, more comfortable sleep.
Material
Hybrid
Trial Period
120 nights
Shipping Method
Free shipping minus HI and AK
Firmness
Medium-firm: 6.5/10
Warranty
10-year warranty
Price Range
$$$$$
Brooklyn Bedding Titan Plus Luxe

This specially-designed hybrid is made for heavier sleepers to help them get better, more comfortable sleep.
Material
Hybrid
Warranty
10-year warranty
Firmness
Medium-firm: 6.5/10
Shipping Method
Free shipping minus HI and AK
Trial Period
120 nights
Price Range
$$$$$

Brooklyn Bedding Titan Plus Luxe
This specially-designed hybrid is made for heavier sleepers to help them get better, more comfortable sleep.
Material
Hybrid
Firmness
Medium-firm: 6.5/10
Trial Period
120 nights
Warranty
10-year warranty
Shipping Method
Free shipping minus HI and AK
Price Range
$$$$$
Why the Titan Plus Luxe Earned Best Mattress for Heavy People with Shoulder Pain
Sleepers over 200 pounds who deal with shoulder pain are going to need a mattress soft enough to alleviate their pain, but with enough firmness to reliably support them. Luckily, Brooklyn Bedding’s Titan Plus Luxe mattress was designed with heavier folks in mind, resulting in a mattress that exemplifies these two qualities.

It’s a step up in price from the original Titan Plus mattress, but better suited for sleepers seeking pain management; the added foam comfort layer should provide plush cushioning around the shoulders.
What’s the Titan Plus Luxe Made Of?
The Titan Plus Luxe is 13 inches thick with five total layers. For a fee, you can add a cooling GlacioTex™ cover to the mattress top.
Inside the Titan Luxe Plus
- Layer 1 – Quilted Top (1 inch). Quilted gel foam cushions your body and reforms easily when you get back up.
- Layer 2 – TitanFlex™ Comfort Foam (2 inches). This medium-feel foam reacts quickly to your body’s movements for specialized contouring.
- Layer 3 – Energex™ Transition Foam (1 inch). This transitional foam layer ensures you don’t sink too far into the mattress.
- Layer 4 – TitanCore Coils (8 inches). TitanCore coils are encased to react individually to your movements, reducing the risk of motion transfer. The edges feature tight rows of higher-gauge coils for reinforcement.
- Layer 5 – Base Foam (1 inch). A dense foam base supports the coils and the rest of the mattress.
Our Take: “As a plus-sized individual myself, something that I really appreciated about this mattress was that it generously supported my weight.” – Sean, Sleep Advisor Mattress Tester
What We Liked
- Can accommodate smaller sleepers – Most mattresses designed for heavyweight sleepers don’t take into account that they may have a partner who’s a different size, thus rendering it too uncomfortable for some. Emma was surprised by the bed’s ability to counteract this: “I’ve tried out a number of mattresses that are built for sleepers who are plus-sized, and generally I find that the mattresses are too firm for my body type and weight. However, the [Titan Plus Luxe] was not like that at all. I find myself very comfortable on this mattress.”
- Keeps cool – Gel-infused foams and individually wrapped coils contribute to this bed’s temperature-regulating properties. Air should flow through the mattress, preventing hot sleepers from overheating.
Potential Drawbacks
- Higher price point – The Luxe comes in at a higher price than its original counterpart, but we think it’s worth the little splurge. Before sales, a queen will run you about $1,600.
- Hard to move – My fellow testers noticed this bed was difficult to move around the studio. It’s hefty, so you’re going to need an extra pair of hands.
Still thinking? Read our Titan Plus Luxe review or our article on the best mattresses for plus-size people.
Saatva Classic – Best Mattress for Back and Shoulder Pain
Saatva Mattress

Coil-on-coil structure gives this elevated innerspring bed a luxurious feel and versatility.
Material
Innerspring
Trial Period
365 nights
Shipping Method
Free white glove delivery
Firmness
Multiple firmness options
Warranty
Lifetime warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Saatva Mattress

Coil-on-coil structure gives this elevated innerspring bed a luxurious feel and versatility.
Material
Innerspring
Warranty
Lifetime warranty
Firmness
Multiple firmness options
Shipping Method
Free white glove delivery
Trial Period
365 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.

Saatva Mattress
Coil-on-coil structure gives this elevated innerspring bed a luxurious feel and versatility.
Material
Innerspring
Firmness
Multiple firmness options
Trial Period
365 nights
Warranty
Lifetime warranty
Shipping Method
Free white glove delivery
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Pain
This bed is perfect for anyone suffering from back pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Why the Saatva Classic Earned Best Mattress for Back and Shoulder Pain
If you’re looking for a bed that tackles both back and shoulder pain, look no further than the Saatva Classic. This luxury mattress boasts two innerspring units to bring major support, alongside a plush pillow top for pressure relief.
There’s also plenty of lumbar-specific features within its layers: a foam lumbar crown, a spinal wire, and zoned coils all work to promote proper alignment. All of these features amount to lots of useful lift beneath your lower back while your shoulders get plenty of cozy cushioning at the top of the bed.

What’s the Saatva Classic Made Of?
The Saatva Classic comes in two heights—11.5 or 14.5 inches—and three possible firmness levels: Plush Soft, Luxury Firm, or Firm.
Inside of the Saatva
- First Coil Layer. The first coil layer contains individually wrapped coils arranged in zones to keep you properly lifted.
- Euro Pillow Top. Three inches of quilted foam start out the bed with plush pressure relief, including firmer quilting in the center to support the lower back. The organic cotton cover is also treated with an antimicrobial Guardin® treatment to ensure cleanliness.
- Lumbar Crown. A crest of foam in the mattress center also aids in supporting the lower back.
- Spinal Wire. Within the two coil units is a wire designed to guide your body into the correct alignment.
- Second Coil Layer. The second coil layer features more traditional interconnected coils to enhance the bed’s support and durability.
- Foam Rails. Along the perimeter, a series of foam rails reinforce the edges of the bed.
My Take: The Saatva gets an A+ when it comes to keeping your body in the right alignment, which helps minimize pain in sensitive areas.
What I Liked
- Customizable – Two heights and three firmnesses mean there’s plenty of combinations to satisfy a wide range of customers. You should be able to customize the bed for the best chance at minimizing your pain.
- White glove delivery – With all of its mattresses, Saatva offers free white glove delivery. This entails a team of experts coming to your residence to set up your mattress and take away your old one, hassle-free.
Potential Drawbacks
- Returns cost extra – Though you get pretty swanky delivery, taking the mattress back is a different story. It’ll cost you $99 to return the bed if you change your mind.
- Could isolate motion better – The Saatva Classic stifled some small movements, like shifting around, but you’re probably going to notice bigger ones, like a partner or pet getting in and out of bed.
Find out more: Check out our dedicated Saatva Classic review, or consider our list of the best mattresses for back pain.
Bear Elite Hybrid – Best Hybrid Mattress for Shoulder Pain
Bear Elite Hybrid Mattress

With coils and foam layers, this Bear mattress offers zoned support without taking away from the pressure-relieving properties. The bed also has copper infusions to ensure the best cooling for hot sleepers and athletes.
Material
Hybrid
Trial Period
120 nights
Shipping Method
Free shipping
Firmness
Medium-firm: 6/10
Warranty
Lifetime Warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Bear Elite Hybrid Mattress

With coils and foam layers, this Bear mattress offers zoned support without taking away from the pressure-relieving properties. The bed also has copper infusions to ensure the best cooling for hot sleepers and athletes.
Material
Hybrid
Warranty
Lifetime Warranty
Firmness
Medium-firm: 6/10
Shipping Method
Free shipping
Trial Period
120 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.

Bear Elite Hybrid Mattress
With coils and foam layers, this Bear mattress offers zoned support without taking away from the pressure-relieving properties. The bed also has copper infusions to ensure the best cooling for hot sleepers and athletes.
Material
Hybrid
Firmness
Medium-firm: 6/10
Trial Period
120 nights
Warranty
Lifetime Warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Hip Pain
This bed is perfect for anyone suffering from hip pain.Back Sleeping
Ideal for lightweight, average weight, and heavyweight back sleepers.Stomach Sleeping
Ideal for lightweight and average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Why the Bear Elite Hybrid Earned Best Hybrid Mattress for Shoulder Pain
The Bear Elite Hybrid is on the softer side when it comes to hybrid mattresses, so it should work well for folks with shoulder pain. The foam layers on top provide a nice buffer, allowing you to sink in comfortably while remaining supported by the coils on the bottom.
This bed also stands out from typical hybrid mattresses because of the innovative fiber in its cover. Celliant® reportedly promotes muscle recovery by converting excess heat into infrared energy, a boon for both hot sleepers and athletes alongside anyone with shoulder pain.

What’s the Bear Elite Hybrid Made Of?
The Bear Elite Hybrid is 14 inches tall and has five layers.
Inside the Bear Elite Hybrid
- Layer 1 – Cover. A mix of Phase Change Material and Celliant® fibers keep it cool at the mattress surface.
- Layer 2 – Copper-Infused Memory Foam. Copper draws away unwanted heat and bacteria while foam cushions your curves.
- Layer 3 – Dynamic Transition Foam. This responsive layer eases you into the mattress without letting you sink in too far.
- Layer 4 – Coils. Zones of individually encased coils maintain support, including along the edges of the mattress.
- Layer 5 – High-Density Support Foam. A sturdy and durable foam base layer.
Our Take: “One of the things that I liked the most about the Bear Elite Hybrid was that it contoured to my shoulders really, really well.” – Stuart Petty, Sleep Advisor Mattress Tester
What We Liked
- Targeted support – Zoning in the innerspring unit should ensure the right equilibrium of lift and give, resulting in a neutral posture—and, hopefully, less pain.
- Super cooling – During testing, we noticed the Bear Elite Hybrid was super cool—no doubt thanks to its extra-cooling cover, breathable foams, and individually wrapped coil layer that keeps air flowing through the bed.
Potential Drawbacks
- Could get too cool – The Bear Elite Hybrid can get chilly for some folks, as my fellow tester Sydney noted: “I prefer a warmer bed to sleep in. But if you’re already a hot sleeper, prefer your room cold, your mattress cold, this bed’s perfect for you.”
- Too plush for some – If you weigh over 200 pounds or prefer to sleep on your stomach, the Bear Elite Hybrid might not be the best choice for you, since it’s a softer hybrid.
Looking for more? Check out our Bear Elite Hybrid review or our picks for the best hybrid mattresses.
Nolah Signature 12″ – Best Soft Mattress for Shoulder Pain
Nolah Signature 12″ Mattress

Shoppers interested in a hybrid feel should find this Signature bed a perfect choice for their needs.
Material
Foam
Trial Period
120 nights
Shipping Method
Free shipping
Firmness
Soft: 4/10
Warranty
Lifetime warranty
Price Range
$$$$$
Nolah Signature 12″ Mattress

Shoppers interested in a hybrid feel should find this Signature bed a perfect choice for their needs.
Material
Foam
Warranty
Lifetime warranty
Firmness
Soft: 4/10
Shipping Method
Free shipping
Trial Period
120 nights
Price Range
$$$$$

Nolah Signature 12″ Mattress
Shoppers interested in a hybrid feel should find this Signature bed a perfect choice for their needs.
Material
Foam
Firmness
Soft: 4/10
Trial Period
120 nights
Warranty
Lifetime warranty
Shipping Method
Free shipping
Price Range
$$$$$
Why the Nolah Signature Earned Best Soft Mattress for Shoulder Pain
The Nolah Signature is one of the softest beds on this list, coming in at a 4 out of 10 on our firmness scale. If you’re looking for a plush foam bed that’ll cradle your shoulders and help prevent pain, it should get the job done.
The bed features Nolah’s proprietary AirFoam™—a porous, breathable foam that has all the cushioning comfort of memory foam, without the penchant for overheating. Enjoy the pleasant sink and hugging pressure relief of the bed’s surface while remaining supported by the firmer foams underneath.

What’s the Nolah Signature Made Of?
The Nolah Signature is 12 inches tall. It’s made up of five layers.
Inside the Nolah Signature
- Layer 1 – Organic Cotton Cover. Organic cotton makes for a naturally breathable and soft cover.
- Layer 2 – Plush AirFoam™ (2.5 inches). A soft layer of cooling AirFoam™ provides cushioning at the mattress surface.
- Layer 3 – Active Response Foam (1.5 inches). This reactive layer of foam adapts easily to your shape and movements.
- Layer 4 – Firm AirFoam™ (1 inch). A firm layer of AirFoam™ keeps the upper comfort layers supported.
- Layer 5 – High-Density Foam Foundation (7 inches). A dense, stable foam base rounds out the mattress.
Our Take: “The Nolah Signature is a softer mattress, so it’s going to excel for side sleepers who want pressure relief in their shoulders.” – Emma Mattei, Sleep Advisor Mattress Tester
What We Liked
- Cool for foam – Innovations in the Nolah Signature’s all-foam construction make it so it won’t overheat like other foam beds. You can thank the cotton cover and breathable foam layers for the bed’s ability to regulate temperature.
- Dampens motion – The thick foam layers help to absorb movements and prevent them from traveling across the mattress surface.
Potential Drawbacks
- Lackluster edges – There’s no special reinforcement along the Nolah Signature’s perimeter, and this unfortunately results in edges that sag when they bear a lot of weight. Stick to the center of the mattress.
- Not for heavy folks – In my opinion, the Nolah Signature is far too soft to feel comfortable for sleepers over 200 pounds. They’re going to sink right through the top layers and meet that uncomfortable firm foundation on the bottom.
Find out more: Read our full Nolah Signature review, and peruse our recommendations for the best soft mattresses.
Brooklyn Bedding Sedona Elite – Best Firm Mattress for Shoulder Pain
Brooklyn Bedding Sedona Elite Mattress

This hand-crafted hybrid model should deliver a premium sleeping experience.
Material
Hybrid
Trial Period
120 nights
Shipping Method
Free shipping
Firmness
Medium: 5.5/10
Warranty
10-year warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Brooklyn Bedding Sedona Elite Mattress

This hand-crafted hybrid model should deliver a premium sleeping experience.
Material
Hybrid
Warranty
10-year warranty
Firmness
Medium: 5.5/10
Shipping Method
Free shipping
Trial Period
120 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.

Brooklyn Bedding Sedona Elite Mattress
This hand-crafted hybrid model should deliver a premium sleeping experience.
Material
Hybrid
Firmness
Medium: 5.5/10
Trial Period
120 nights
Warranty
10-year warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Couples
This bed has great motion isolation so you will not feel your partner tossing and turning at night.Side Sleeping
Ideal for lightweight and average weight side sleepers.Financing Options
Financing options are available for this mattress.
Why the Brooklyn Bedding Sedona Elite Earned Best Firm Mattress for Shoulder Pain
Firm mattresses can be tough if you’re dealing with shoulder pain, since a bed that lacks pressure relief may exacerbate existing aches. The Brooklyn Bedding Sedona Elite is a great option if you’re looking for a medium-firm bed that’ll bring the support without the added stress to your shoulder.
Two coil units give the bed its supportive edge, including a miniature coil unit closer to the mattress surface for more precise pressure relief and reactivity.

What’s the Brooklyn Bedding Sedona Elite Made Of?
The Sedona Elite by Brooklyn Bedding is made up of six layers, making it 14 inches thick.
Inside the Brooklyn Bedding Sedona Elite
- Layer 1 – Cooling Cover. GlacioTex™ cooling technology regulates temperature at the mattress surface.
- Layer 2 – Copper Memory Foam (1.5 inches). This memory foam layer features copper for added cooling and antimicrobial properties.
- Layer 3 – Micro-Wrapped Coils (2.5 inches). A layer of miniature coils keep air flowing through the mattress while its springs respond individually to your movements.
- Layer 4 – Transition Foam (1.5 inches). A dense foam layer transitions into the coil unit underneath.
- Layer 5 – Ascension® Coils (8 inches). The second layer of larger encased coils gives the bed its solid support.
- Layer 6 – Foam Base (1 inch). A high-density foam foundation.
Our Take: “The Brooklyn Sedona mattress employs several layers of comfortable memory foam which gently cradle your body as you lie down, providing great pressure relief. All while the system of individually encased coils provides superior support.” – Emma, Sleep Advisor Mattress Tester
What We Liked
- Plenty cool – The Sedona Elite is a far cry from its Arizona namesake, thanks to its temperature-regulating ability. A cooling cover, breathable foams, and two innerspring units all contribute to the bed’s cool feel.
- Big bounce – Two coil units mean double the bounce. Moving around on the mattress surface should feel easy for any combination sleepers or sexually active couples.
Potential Drawbacks
- Doesn’t dampen motion – The aforementioned springiness isn’t a good match for light sleepers, since movements won’t be absorbed as well as they would on an all-foam bed.
- Not a budget option – A queen-size model of this mattress will run you about $2,300. Coupons can often bring this price down by a few hundred dollars, though.
Still looking? Check out our dedicated Brooklyn Bedding Sedona Elite mattress review or the best firm mattresses.
Birch Natural – Best Organic Mattress for Shoulder Pain
Birch Natural Mattress

An eco-friendly bed that pairs latex and individually wrapped coils for a versatile hybrid sleep experience.
Material
Hybrid
Trial Period
100 nights
Shipping Method
Free shipping
Firmness
Medium-firm: 6.5/10
Warranty
25-year warranty
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Sleeping
Ideal for average weight back sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Birch Natural Mattress

An eco-friendly bed that pairs latex and individually wrapped coils for a versatile hybrid sleep experience.
Material
Hybrid
Warranty
25-year warranty
Firmness
Medium-firm: 6.5/10
Shipping Method
Free shipping
Trial Period
100 nights
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Sleeping
Ideal for average weight back sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.

Birch Natural Mattress
An eco-friendly bed that pairs latex and individually wrapped coils for a versatile hybrid sleep experience.
Material
Hybrid
Firmness
Medium-firm: 6.5/10
Trial Period
100 nights
Warranty
25-year warranty
Shipping Method
Free shipping
Price Range
$$$$$
We recommend this mattress for the following sleeper types:
Hot Sleepers
If you often overheat while you sleep, this mattress should help you stay cool.Seniors
This bed is perfect for senior sleepers.Back Sleeping
Ideal for average weight back sleepers.Stomach Sleeping
Ideal for average weight stomach sleepers.Financing Options
Financing options are available for this mattress.
Why the Birch Natural Earned Best Organic Mattress for Shoulder Pain
Environmentally conscious shoppers with shoulder pain should find their match in the Birch Natural. This bed features plenty of green materials, including organic cotton, organic wool, and naturally derived latex.
This construction results in a comfortable and bouncy medium-firm bed that should suit a wide variety of sleepers, as well as resist infiltration by germs and allergens..
What’s the Birch Natural Made Of?
The Birch Natural has six layers and stands 11 inches tall.
Inside the Birch Natural
- Layer 1 – Organic Cotton Cover. GOTS-certified organic cotton swaddles the top of this bed for a soft and breathable surface.
- Layer 2 – Fire Retardant Organic Wool. The first layer of organic wool acts as a fire barrier—without the use of any synthetic chemicals.
- Layer 3 – Organic Wool. The next layer of organic wool provides insulating comfort.
- Layer 4 – Talalay Latex. This natural latex layer bounces back for uniquely responsive pressure relief.
- Layer 5 – Coils. Individually wrapped steel coils bring lift and motion isolation. The long sides of the mattress are reinforced for stronger edges.
- Layer 6 – Base Wool Layer. A final layer of fire-protective wool acts as the mattress foundation.
Our Take: “The organic cotton cover is soft to the touch, and the wool layers are comfortable and cozy to lie down on.” – Stuart, Sleep Advisor Mattress Tester
What We Liked
- Strong edges – Thanks to reinforcement along the longer edges of the bed, you can make use of the full mattress space.
- A pleasant firmness – Latex and springs give this bed a lifted feel, rather than the swaddling sink of foam. This profile should do well when it comes to promoting alignment—especially if you sleep on your back or stomach.
Potential Drawbacks
- Too firm for some – The Birch’s firmness might be too much for some lightweight side sleepers. If this is the case for you, Stuart recommended adding on a mattress topper for extra cushion.
- Organic construction, high price – These high-quality natural and organic materials translate into high production costs, which then lead to high sales costs. Though it’s worth every penny, be aware that the Birch Natural will run you over $3,000 before any sales.
Want to know more? Read our Birch Natural review, or find out our picks for the best organic mattresses.
What to Look for in a Mattress for Shoulder Pain
When you’re dealing with shoulder pain, it’s critical you get something that will give you some relief and not exacerbate your discomfort. While you might think buying a mattress is just about the price point, plenty of important factors can impact how the bed performs for you.
Type of Mattress

Innerspring Mattresses
Innerspring mattresses are a classic design that generally relies on a layer of coils and a thin comfort layer above them. While they have many benefits, innerspring beds don’t generally offer much in the way of pressure relief, which means they could wind up creating more pressure and discomfort. For this reason, I would say they’re not the best option for shoulder pain.
Hybrid Mattresses
Hybrids take the coil design of innersprings and add foam layers on top for added pressure relief and comfort. This might include memory foam, latex foam, poly foam, or a combination of these materials. Hybrid mattresses can be a good option for those who love the bounciness and support of innerspring models but need added comfort and contouring for the shoulders.
Memory Foam Mattresses
Memory foam is popular because it excels at pressure relief, which is why beds containing this material could be great for those struggling with shoulder pain. These beds often feature memory foam on top, followed by denser poly foam layers for support. However, this material is often found in hybrid mattresses too. Another perk to these beds that I like is they’re typically more affordable than hybrid and latex models.

Latex Mattresses
Latex beds might not be as well-known as memory foam or innerspring designs, but the material is a great alternative for those seeking something for pressure relief but without some of the pitfalls you might find with memory foam models. For example, latex sleeps cooler and is more responsive. Additionally, many latex mattresses are more eco-friendly, which is great for those looking for something that’s good for their shoulder pain and the planet.
How We Test The Best Mattresses for Shoulder Pain
Sleeping Position

- Back sleepers will want a mattress that supports their lower back but also provides cushioning for their shoulder area, especially if they’re experiencing pain. When testing for back sleeping, I focus on how well the bed evenly distributes my weight and supports my lower back.
- Side sleepers will want to avoid sleeping on the specific side that’s in pain. When you lie on your side, most of your weight is concentrated in the shoulder, as well as the hip, so it’s best to avoid undue pressure that can worsen your pain. When testing mattresses for side sleeping, I evaluate how well the mattress does at relieving any pressure in my shoulder and hip while maintaining overall body support.
- With stomach sleepers, it’s vital to keep the hips lifted so the lower back doesn’t arch. However, if you’re dealing with shoulder pain, you still want something that’s soft against the shoulders. I’m a stomach sleeper, so I love testing how each bed feels in this position, focusing mostly on how well it keeps my hips elevated and body aligned.
- Combination sleepers need a quick-responding bed that will allow them to change positions more easily, coupled with a medium-firm feel that accommodates multiple sleep positions. When testing beds for combination sleepers, I like switching between positions to see how easily I can move and adapt.

Weight/Body Type of Sleepers
Your weight and body type can impact how a mattress performs. For example, people who are heavier are going to sink into a mattress more than lightweight people.
We generally consider lightweight sleepers below 130 pounds, average-weight sleepers between 130 and 230 pounds, and heavyweight sleepers over 230 pounds. I’m average-weight, but because we like to consider all weight groups, I also have my colleagues Loren Bullock and Spencer Bland test beds as lightweight and heavyweight sleepers, respectively.
- Lightweight sleepers often find softer to medium-firm beds the best, depending on their sleep position. For instance, a lightweight side sleeper will likely feel best on a softer mattress, whereas a lightweight stomach sleeper would probably need something medium-firm for the added midsection support.
- Average-weight sleepers, like myself, often respond best to medium-firm beds since these are usually the most accommodating for this group. Then again, sleep position will also factor in here.
- Heavier sleepers will often find medium-firm to firm beds more accommodating, though again, this will also depend on their sleeping position.

Firmness
Here at Sleep Advisor, we rate mattress firmness on a scale of 1-10, with 10 being the absolute firmest feel. Most mattresses fall within the medium range since this feel works for most people. If you have shoulder pain, though, you may prefer something with a slightly softer feel than you otherwise normally would, especially if you’re a side sleeper.
- Anything below is a 5 is considered soft
- Anything between a 5 and 6.5 is considered medium or medium-firm
- Anything a 7 or higher is considered firm
Which Firmness is Best for Shoulder Pain?
Generally speaking, it’s best to choose a softer mattress that can contour and cushion your shoulders to minimize any pressure that might worsen your pain. A firmness rating of 5 and 6 on our scale should be good for shoulder pain, in my opinion, but again, consider your preferred sleeping position as well because a bed that’s too soft can be a nightmare for the hips of a stomach sleeper.
Cooling
If you run hot at night, a cooling mattress is worth the investment, especially if you have pain.
The reason for this is that poor sleep can worsen pain symptoms1 so you want a mattress that’s going to give you the best chance of getting a good night’s rest.
At Sleep Advisor, we use a heat gun to measure how well a mattress regulates temperature. I start by checking the initial surface temperature. Next, I’ll check the temperature after five minutes of lying on the bed to see how much the mattress heats up. I then repeat this step to see how hot it gets after 10 minutes.

We consider a mattress good at temperature regulation when its initial temperature goes up no more than 5 degrees — bonus points if the temperature doesn’t change between the 5- and 10-minute rest tests.
How Important is Spinal Alignment for Shoulder Pain?
According to a physical therapist Palak Shah, “Poor spinal alignment, particularly in the cervical (neck) and thoracic (upper back) regions, can directly impact shoulder mechanics.”
Shah goes on to say, “It’s important to consider the biomechanical and neural interconnections of the human body. Poor spinal alignment leads to poor spine mobility, leading to poor shoulder mobility, which then leads to stress and overstrain, eventually leading to imbalance. This imbalance leads to pain, which leads to decreased movement, and the cycle goes on.”
Motion Isolation
Motion isolation is a great quality in mattresses for couples since it helps keep movements from transferring to the other side of the mattress.
If you have shoulder pain, it might take you longer to fall asleep, and the last thing you want is to suddenly awaken because your partner got out of bed.
Memory foam beds are the best for this, but memory foam hybrids can do well too.

I use a seismograph app to see just how much movement transfers from one side to the other. Small waves indicate good motion isolation, but big waves mean there is plenty of transfer. I also have a teammate move on the other side of the bed to test motion isolation to really get a feel for it as a co-sleeper.
Ease of Movement and Responsiveness
If you are in pain, having a bed that makes it easier to move around should be a big help. Often, beds that are quick-responding help facilitate movement more easily. Coils and latex are examples of responsive materials. Conversely, memory foam is slow-moving.
More often than not, if I found poor motion isolation readings from the seismograph app, I knew I could expect good bounce. I further test this with lacrosse and weighted medicine balls and by jumping on the bed. This way, I can manually gauge how quickly materials come back to shape after you apply weight to them.
Pressure Relief & Contouring

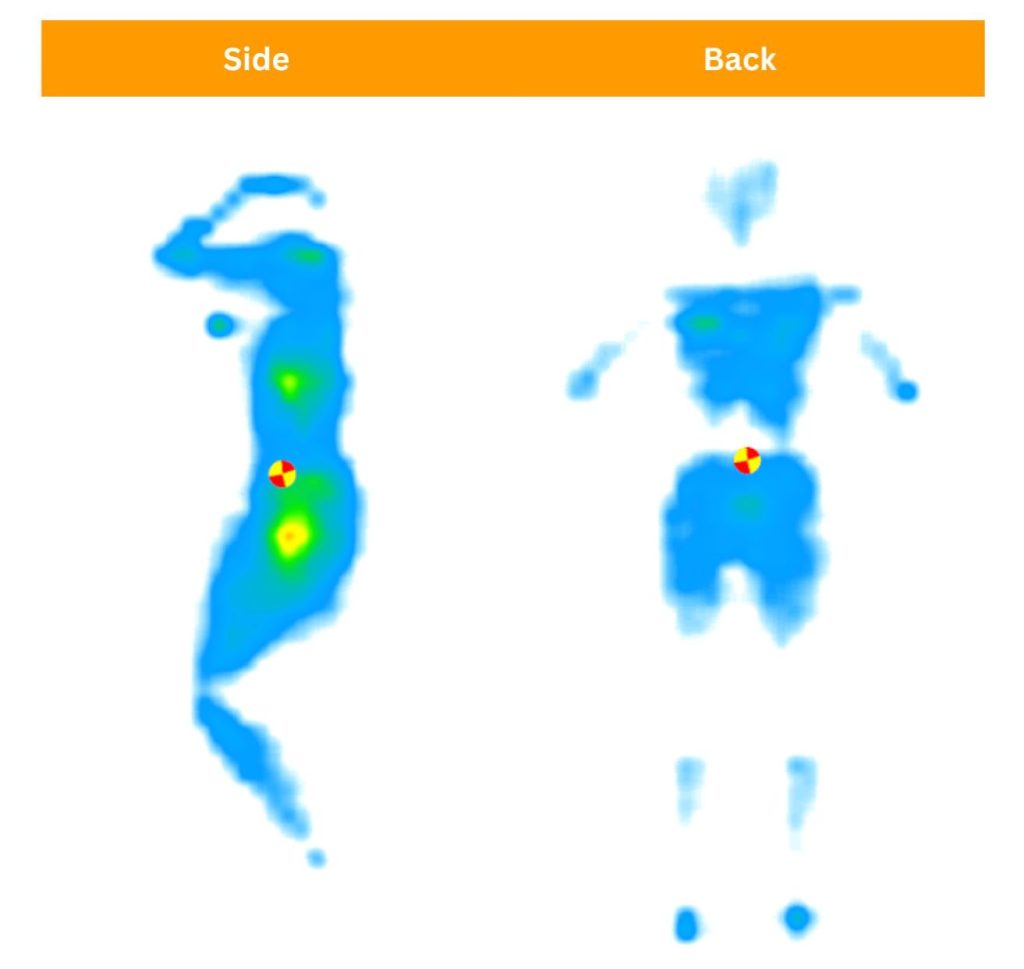
This is vital for those with shoulder pain. Pressure relief means that the mattress helps take pressure off sensitive areas through materials like memory foam that contour around the sleeper’s curves.
We use a pressure map to see if there’s any buildup when lying on the mattress. All three of us switch between side and back sleeping to see how well a mattress relieves pressure for all three weight/body type groups. Red and yellow readings show pressure buildup, while green and blue readings show pressure relief.
Shop the Best Mattresses for Pressure Relief here.
Edge Support
Edge support measures how supportive the bed is along the sides. If you’re someone who likes to sleep at the edge, needs more useable surface space, or often sits on the edge to get ready, this is a helpful mattress quality.
We test this the old-school way by sitting at the edge for a minute to tie our shoes and then we lie near the edge for another minute. This way, we can gauge any sinking and slipping. I would say a mattress has good edge support when sinking and slipping are minimal.
Material Quality and Durability
A mattress is a significant investment, and you want something that’s going to hold up well over time. Generally, mattresses have a lifespan of roughly seven years, but this can go higher or lower depending on the quality of the mattress and how the bed is maintained.
Look for beds from reputable brands that are known for providing quality products. While it’s tempting to go with the cheapest option out there, these likely won’t last you long and could cost you more since you’ll need to invest in a new bed sooner. As far as durability, latex often lasts the longest, but it is also more expensive, so you’ll have to weigh the pros and cons of this with your budget.
Looking for more durable beds? Explore our picks for the best mattresses that don’t sag.
Sex and Couples
Whether a mattress is good for sex and couples in general depends on a few factors. I usually like to consider motion isolation for couples, especially those with different sleeping habits. However, edge support and cooling are also important, allowing you to use the entire surface of the bed without overheating.
As for sex, most people look at how bouncy a mattress is, and rightly so. However, there’s more to it than just going for the bounciest bed. In my opinion, bounce is good for sex, but too much of it might be counterproductive for motion isolation. For this reason, it’s good to have some balance between the two.
Budget
Understandably, your budget is a top concern going into your mattress search. As mentioned, going with extremely cheap mattresses isn’t always smart since you won’t get something substantial. However, that doesn’t mean you need to break the bank either.
Generally, a good budget-friendly range for Queen-size beds is around $1,000-$1,500.
To help save you money, we suggest shopping online, especially during big mattress holiday sales, since companies will slash prices even more. This means you can save significantly on a well-made bed.
Learn More: Best Budget Mattress and Best Mattress Under $1,000
What Causes Shoulder Pain?
Bursitis
Bursitis2 occurs when fluid around your joints becomes inflamed and leads to pain. While people might experience it during the day, it often feels worse at night because of added pressure (another reason why a pressure-relieving mattress is vital). Bursitis pain can appear suddenly or develop over time3.
Tendonitis
According to the Cleveland Clinic4, tendonitis is when the tendons, which are the connective tissues between your muscles and bones, become inflamed. They add that tendonitis can often happen due to repetitive activities. For example, pitchers or regular swimmers might experience this from the repetitive use of their shoulders.
Arthritis
Arthritis is when the joints become inflamed and tender5, typically resulting in stiffness and pain. According to the Mayo Clinic, the two most common forms of arthritis are rheumatoid arthritis (RA) and osteoarthritis. They add that some of the risk factors for arthritis include advanced age, genetics, being female, experiencing a previous joint injury, and excess weight.
Discover our picks for the best mattresses for arthritis.
Injury
Shoulder pain can also arise from an injury. The American Academy of Orthopaedic Surgeons6 reports that injuries often involve muscles, ligaments, and tendons but in certain cases, a bone fracture or break may occur. Some of the signs they say to look out for include stiffness, whether it feels like the shoulder may pop out, and a lack of strength.
How Does Shoulder Pain Affect Sleep?
Shoulder pain can certainly make it more challenging to sleep because it can be hard to find a comfortable position, and the pain can be distracting. Above, I mentioned that a poor night’s sleep can also exacerbate pain sensitivity, ultimately creating a problematic cycle.1 For this reason, it’s important to find the best solutions to help you get better quality sleep.
How Can a Mattress Help with Shoulder Pain?
The right mattress could be a game-changer in helping you sleep better even with shoulder pain. The following examples are some of the top ways a mattress could help with shoulder pain.
- Pressure relief – A mattress with pressure-relieving materials like memory foam or latex can provide a cushion against your shoulder, cradling the area rather than creating more pressure and worsening your pain.
- Support – A well-made mattress can also promote good body alignment, more specifically, a neutral spine. This can help you feel more comfortable and prevent pain from emerging in other areas, such as the lower back.
- Improved sleep – Of course, the overall takeaway from a good mattress for shoulder pain is better sleep, which can mean reduced pain sensitivity.1
“A mattress can make shoulder pain worse if it’s too firm and creates pressure points, or if it’s too soft and doesn’t offer enough support. But the right mattress, one that’s just the right balance of support and cushioning, can actually help relieve shoulder pain by keeping your spine aligned and reducing pressure. It’s all about finding what feels best for you to get a good night’s sleep without aggravating your shoulders.”
Dr. Raj Dasgupta
Can a mattress topper help if my mattress is causing shoulder pain?
If your mattress is causing shoulder pain because it is too soft or too firm, a topper can help the situation. However, toppers, much like mattresses, require a bit of consideration before you buy them. Think about the thickness and firmness that could modify the feel of your mattress in the right way to mitigate shoulder pain. In my experience, a thicker topper with a medium firmness should work for most people, including those with shoulder pain.
What Is the Best Sleeping Position for Shoulder Pain?
Back Sleeping
Back sleeping is a good position for shoulder pain since your weight is more evenly spread out. With that in mind, it’s also important that your mattress can adequately support your lower back. To help ease pressure on the lower back, you could also try placing a pillow under your knees. If you have an adjustable base, sleeping in the zero gravity position could also help alleviate pressure.
Related: Best Adjustable Beds
Side Sleeping
Orthopedic experts say that side sleeping isn’t ideal for shoulder pain and could sometimes actually cause it7. Most importantly, though, they emphasize taking pressure off the shoulder that’s in pain. If you’re an avid side sleeper, this means you’ll have to switch sides. However, just be careful that your mattress cradles you enough that it doesn’t create pain and pressure in the other shoulder as well.
Combination Sleeping
People with shoulder pain might be more inclined to combination sleeping as they try to find a comfortable position. As mentioned above, combination sleepers should have something conducive to multiple positions, such as a medium-firm mattress with responsive materials to facilitate easier movement.
Stomach Sleeping
Stomach sleeping is another position that might put extra pressure on your shoulders besides not being the best for your lower back. Ideally, your mattress should keep the hips level so that the lower back doesn’t curve. Medium-firm to firm mattresses will generally be best for this sleeping position.
Best Mattress for Shoulder Pain FAQs
Is Memory Foam Good For Shoulder Pain?
Memory foam has excellent contouring and pressure-relieving capabilities, so I think it should be good for shoulder pain. However, be mindful of the mattress’s firmness because memory foam that’s too firm can be painful to sleep on with shoulder pain. Going back to the best firmness for shoulder pain, I’d suggest memory foam that’s soft to medium and medium-firm, depending on your preferred sleeping position and body weight.
Is my mattress causing my shoulder pain?
Yes, orthopedic experts say that sometimes a mattress could cause shoulder pain.7
When this happens, it could be due to sleeping on your side on a mattress that’s too firm, which can create pressure buildup that might eventually lead to pain. Other times, the wrong mattress might end up exacerbating existing pain as well.
What type of mattress topper is best for shoulder pain?
Usually, a softer mattress topper made with memory foam would be best for shoulder pain. The foam can contour the sleeper’s curves to help alleviate pressure buildup. However, if you’re already sleeping on a very soft mattress, it might be helpful to consider a more medium-firm topper that can still provide cushioning but also give you some added support.

Julia Forbes
Lead Product Tester
About Author
Julia is the Lead Reviewer at Sleep Advisor, specializing in testing out mattresses and sleep accessories – she’s in the right line of work, because she loves to sleep.
Stomach Sleeper
Education & Credentials
- Certified Sleep Science Coach
References:
- Levy Anderson, Monica., et al. “Sleep Disturbance and Pain: A Tale of Two Common Problems”. Chest. 2018.
- Williams, Christopher H., Jamal, Zohaib., Sternard, Britni T. “Bursitis”. StatPearls. Last modified July 24, 2023.
- “Bursitis”. Cleveland Clinic. Last modified March 7, 2023.
- “Tendonitis”. Cleveland Clinic. Last modified July 18, 2023.
- “Arthritis”. Mayo Clinic. Last modified August 29, 2023.
- “Common Shoulder Injuries”. American Academy of Orthopaedic Surgeons. Last modified April 2023.
- “Can sleeping positions cause shoulder pain?”. The Centers for Advanced Orthopaedics. 2018.
